Active Recall
FDA Public Warning
Manufacturing Violations
Generic
Learn more about these flags
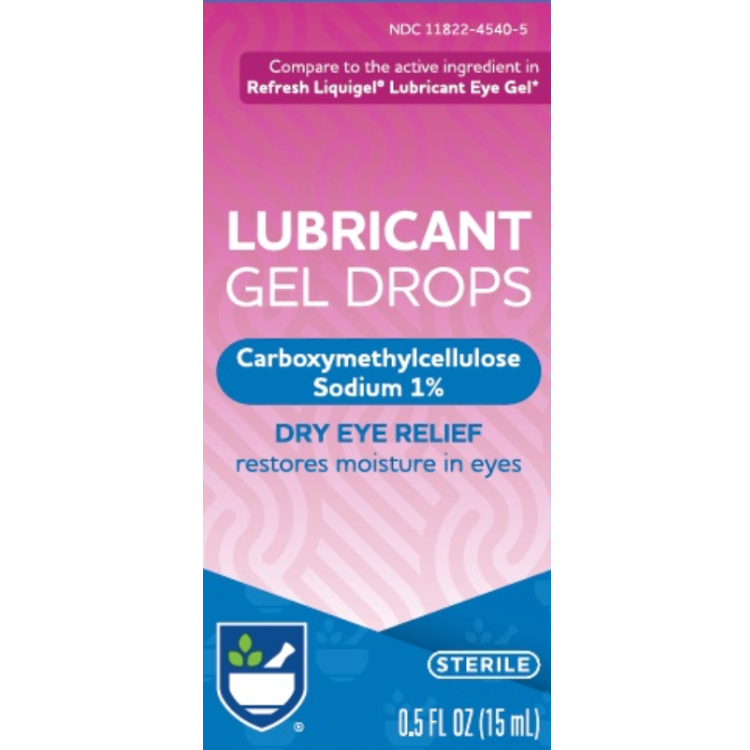
This product also has NDC 76168-351-15 with Velocity Pharma LLC as packager. The box on DailyMed has NDC 11822-4540-5.
Brand:Rite Aid Pharmacy
Generic for:Refresh Liquigel Lubricant Eye Gel
Country of Manufacture:India
Package Type:Multi-dose bottle
Contains preservative(s):Yes
Preservative:Stabilized oxychloro complex
Active ingredient(s):Carboxymethylcellulose sodium 1% per FDA public warning. No FDA-registered ingredient list.
Inactive ingredient(s):Boric acid; calcium chloride; magnesium chloride; potassium chloride; purified water; stabilized oxychloro complex; and sodium chloride. May contain hydrochloric acid and/or sodium hydroxide to adjust pH
NDC(s):11822-9706-5
FDA Action:3/28/24 Warning Letter to Kilitch Healthcare India Limited
11/15/23 Kilitch Healthcare India Limited Issues Voluntary Nationwide Recall of Various Eye Drops for Potential Safety Reasons
10/27/23 FDA warns consumers not to purchase or use certain eye drops from several major brands due to risk of eye infection
11/15/23 Kilitch Healthcare India Limited Issues Voluntary Nationwide Recall of Various Eye Drops for Potential Safety Reasons
10/27/23 FDA warns consumers not to purchase or use certain eye drops from several major brands due to risk of eye infection
Last updated:3-28-24